Description
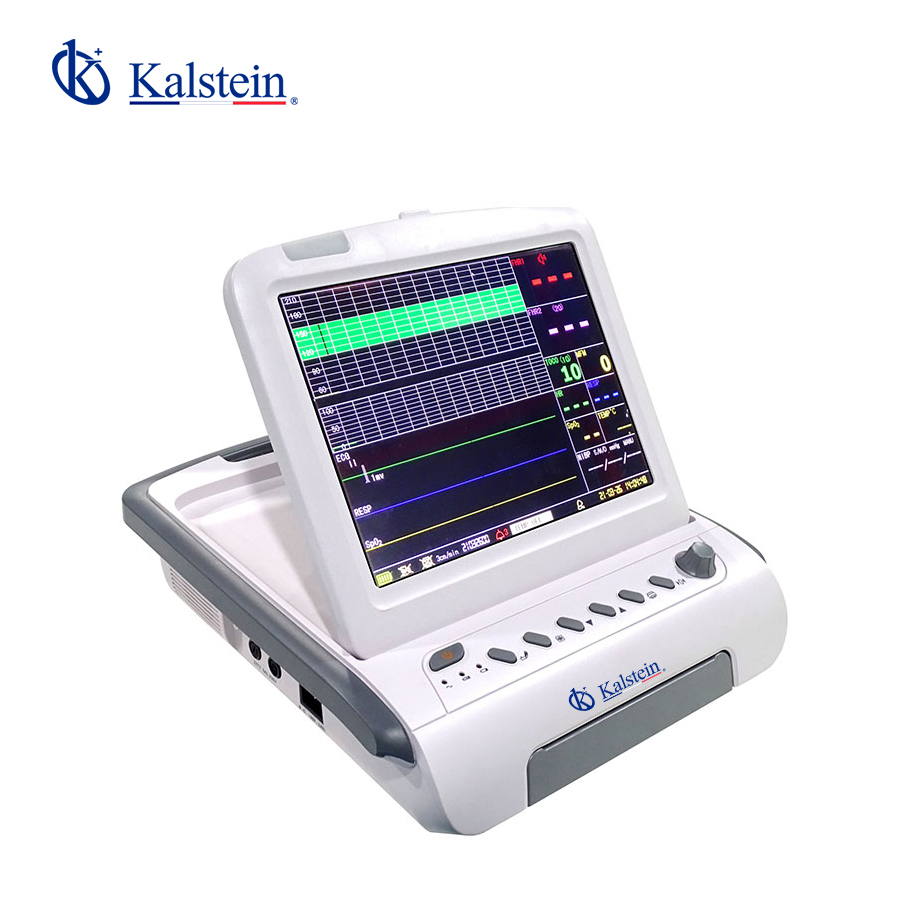
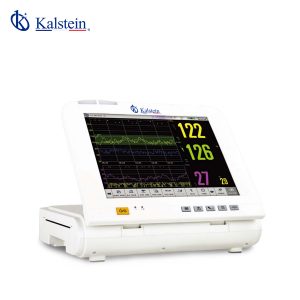
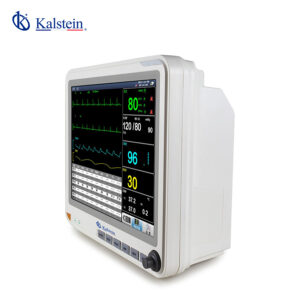
The Doppler Ultrasound Fetal Monitor YR02181 is an exceptional medical device meticulously designed to monitor fetal health parameters with precision throughout pregnancy. Featuring a 12.1-inch folding color TFT screen, capable of displaying up to six waveforms, this monitor provides comprehensive analysis for healthcare professionals. Equipped with standard functionalities such as FHR1, TOCO, and FM, it can also be customized with options such as a fetal stimulator and additional FHR channels. Its accurate measurement capabilities for TOCO, SpO2, HR, NIBP, and temperature are housed in a lightweight and compact design, making it ideal for diverse medical environments. Elevate your clinical practice with the YR02181 by exploring a quote through the Kalstein Plus platform today.
Market Price
The price of the Doppler Ultrasound Fetal Monitor YR02181 varies within a broad range consistent with its advanced capabilities and the reliable performance healthcare professionals seek in high-quality fetal monitoring systems.
Frequently Asked Questions
Q: Can the monitor store and playback data?
A: Yes, the YR02181 is equipped with data storage and playback features, facilitating ongoing tracking of fetal health metrics.
Q: What optional configurations are available?
A: The monitor offers optional features such as a fetal stimulator, a second FHR channel (FHR2), and a mobile trolley for enhanced versatility.
Advantages and Disadvantages
Advantages:
- High-resolution display for superior data visualization.
- Comprehensive monitoring of various parameters for detailed fetal assessment.
- Effortless data storage and retrieval capabilities.
Disadvantages:
- Higher initial cost compared to basic monitors.
- Requires training to fully utilize its advanced features.
Product Usage in the Field
The Doppler Ultrasound Fetal Monitor YR02181 is widely employed in hospitals and clinics to monitor fetal health during prenatal check-ups. Its accurate readings are crucial for the early detection of potential issues, enabling timely medical interventions. Furthermore, its portability and optional trolley enhance operational efficiency by facilitating use across different departments.
Recommendations
For optimal performance, regularly calibrate and maintain the monitor following the manufacturer’s guidelines. Invest in comprehensive training for healthcare staff to maximize the monitor’s capabilities, and consider integrating it with digital record systems for efficient data management.
Features
- 12.1-inch folding color TFT screen
- Maximum 6 waveform display
- Accurate measurement of critical health parameters such as HR, SpO2, NIBP, and more
- Optional wireless connectivity for seamless data sharing
- Lightweight and easily transportable
Technical Specifications
| Model | YR02181 |
| TOCO | Measurement Range: 0-100 units |
| SpO2 Measurement | Measurement Range: 70%~99% Measure Accuracy: ±3% error |
| HR Measurement | Measurement Range: 30bpm~240bpm Measure Accuracy: ±2 bpm |
| NIBP Measurement | Measurement Range SYS 6.7~32.0kPa(50~240mmHg) MEAN: 3.4~26.6kPa(25~200mmHg) DIA: 2.0~24.0ka(15~180mmHg) |
| Temperature | Temperature Arrange:0~50 ºC Resolution: 0.1 ºC Accuracy: 0.1 ºC( excluded the error caused by transducer) |
| Display | LCD shows the FHR trace, TOCO tracd, FM, Doctor Event Mark, Time, Volume etc monitor state, and also it can store and playback. Dimension: 350L×320W×85H(mm) Net Weight: 3.5 kg |